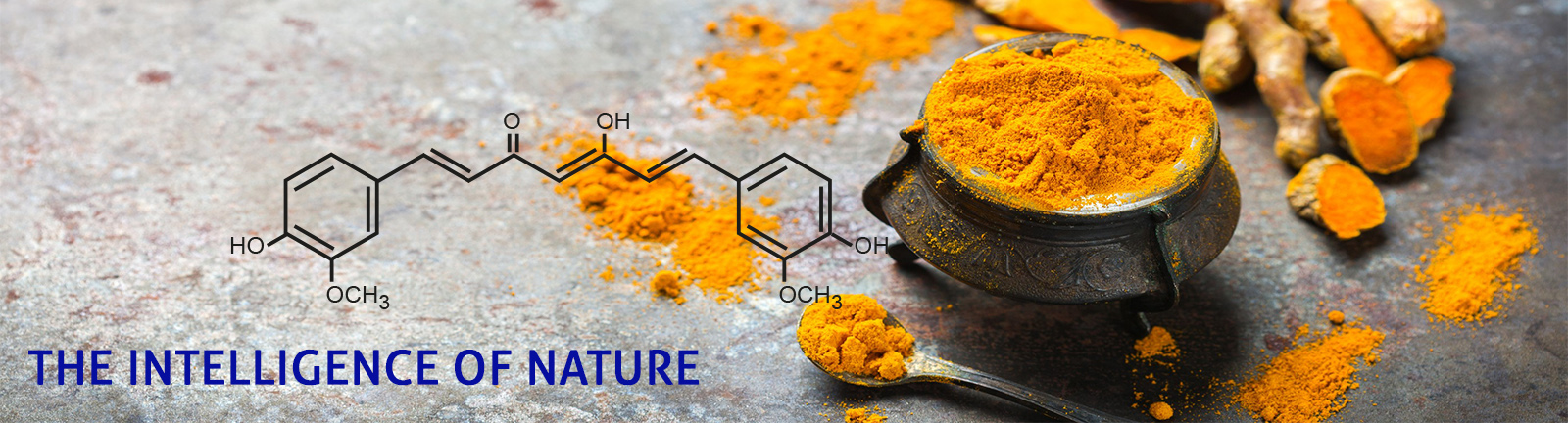
The intelligence of nature made available with high tech
Chronic inflammation lasting for years is considered one of the major causes of cancer. Several studies by Prof. Bharat Aggarwal from the MD Anderson Cancer Center at the University of Texas, USA, have shown that the transcription factor NF-kB plays an important role in the signalling pathway in the regulation of genes involved in carcinogenesis.
Turmeric root (botanical name, Curcuma longa) contains the anti-inflammatory ingredient curcumin. Alongside other mechanisms, curcumin also has a corrective effect on transcription factor NF-kB. According to Prof. Aggarwal, this explains the inhibitory effects curcumin has on the proliferation, invasion, angiogenesis and formation of metastases in cancer. Curcumin is also shown to have a synergistic effect in combination with various chemotherapies, such as gemcitabine, 5-fluorouracil, Temodal, Herceptin or taxanes.
However, curcumin is poorly soluble in water and is thus only absorbed by the body to a small extent after oral ingestion. The new PuranoTec® manufacturing process by Apurano Pharmaceuticals converts curcumin into a soluble form, without the addition of chemical solvents such as ethanol or hydroxypropyl-ß-cyclodextrin.
Using the PuranoTec® production process, Apurano Pharmaceuticals has manufactured the prescription drug CurcumaXan, which contains curcumin and can be obtained in pharmacies via a prescription from your doctor or alternative practitioner. CurcumaXan is a liquid preparation and is used as an oromucosal spray. Since the oral mucosa are highly perfused by blood, curcumin can be absorbed directly into the bloodstream. As a result, the anti-inflammatory substance is available to the body quickly.
For more information, please contact our Sales Department for Doctors and Alternative Practitioners
CurcumaXan – Curcumin in a new form
Turmeric root has been used in medicine for centuries. However, its main ingredient, curcumin, is virtually insoluble in water, and is therefore absorbed only to a small extent via the gastrointestinal tract when administered orally. Even at high doses of up to 1800 mg, plasma level has been show to be very low, with an AUC of 10.8 ng/ml*h over 12 h and a maximum blood concentration of 2.3 ng/ml (Jäger et al. 2014). In addition to the poor water solubility, this is also due to the fact that, after absorption through the intestinal lining, curcumin is then degraded very quickly in the liver by means of the first-pass effect, and thus only part of the already low amount absorbed makes it into the blood. Curcumin is thus only available for a short time in the body. The PuranoTec® manufacturing process converts curcumin into a new dosage form to optimise its pharmacokinetics. The "drug delivery system" used allows curcumin to be absorbed through the oral mucosa, bypassing first-pass metabolism and increasing bioavailability.
CurcumaXan – the first oromucosal mouth spray of a pharmaceutical grade

CurcumaXan® is manufactured as a medicinal product of proven pharmaceutical grade under statutory GMP conditions in a cleanroom up to class A. Therefore, no preservative is necessary to ensure microbial stability. This means the patient and therapist can rely on the drug having a consistent concentration as well as microbial quality over its shelf life. Regular quality controls such as residue analyses, process controls and chemical, physical and microbial stability controls ensure the high quality of this medicinal product. For this reason, the initial quality of the raw materials as well as the final quality of CurcumaXan® are verified using state-of-the-art technology, in accordance with the provisions of the German Medicines Act.
For what indications is there scientific proof of action for curcumin?
The element responsible for the numerous medical effects is predominantly the yellow polyphenol curcumin. Curcuma longa contains curcumin (curcumin I), monodesmethoxycurcumin (curcumin II) and didesmethoxycurcumin (curcumin III). Curcumin is highly antioxidant: it intercepts oxygen and nitrogen radicals and prevents the oxidation of cholesterol. In addition, it inhibits numerous inflammatory parameters such as prostaglandins, cytokines, chemokines, adhesion molecules, growth factors and transcription factors, as shown by numerous studies, including by Mazieiro et al. Due to the modulation of cytokine signalling pathways and its antioxidant effect, curcumin is suitable for use to successfully treat chronic inflammatory and degenerative diseases. Curcumin is also recommended in the current S-3 guideline "Diagnosis and Treatment of Ulcerative Colitis" as a complementary measure alongside mesalazine.
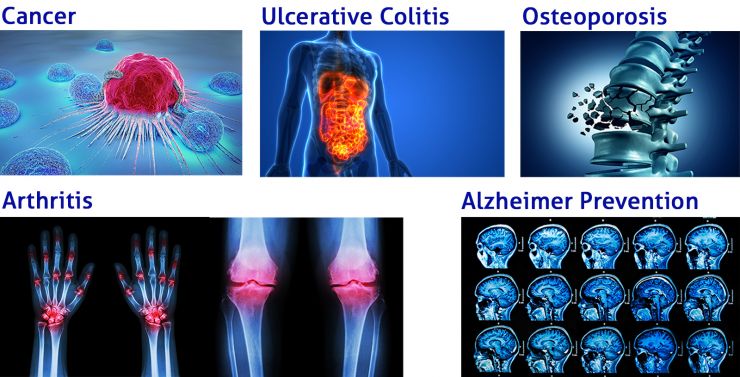
Clinical studies with curcumin preparations have shown positive effects for the following indications, among others:
- Oncological indications
- Arthritis
- Inflammatory bowel disease
- Postoperative inflammation (Gupta et al. 2013).
- Ulcerative colitis (recommendation in accordance with the S3 guideline)
According to the latest studies, curcumins are said to also have a positive effect on cancer. Datta and Banerjee analysed their tumour-inhibiting properties in an in-vitro study. In various cancer cell lines, such as breast cancer and melanoma, curcumin was able to counteract tumour growth by inhibiting phosphorylation processes. A study by Galindo is interesting in this context. Using X-ray crystallography, the researchers were able to show that curcumin influences the enzyme DYRK2 ("dual-specificity tyrosine-phosphorylation-regulated kinase 2), which is involved in cell proliferation and tumour growth, among other things.
How safe is curcumin?

Despite multiple studies, only a few side effects are known of for curcumin. Only in studies using very high turmeric concentrations have side effects such as diarrhoea, headaches and rashes been observed (Lao et al. 2006). The new pharmaceutical form of CurcumaXan® allows one to achieve higher blood levels of the active ingredient while using significantly lower doses than in the case of oral administration. This lower exposure also decreases the dose-related risk of side effects. Curcuma longa and curcumin are always contraindicated in the case of biliary disorders, as curcumin stimulates the gallbladder (EMEA 2009).
References
EMEA (2009) Assessment Report on Curcuma longa L. Rhizoma. In Committee on herbal medicinal products.
Mazieiro et al. (2018), Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases?, J Med Food. 2018 Jun 29. doi: 10.1089/jmf.2017.0146
Datta et al. (2018), Orthogonal self-assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells, PNAS August 7, 2018 115 (32) 8087-8092
Banerjee (2018), Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2, PNAS August 7, 2018 115 (32) 8155-8160
Galindo et al. (2018), Crystal structure reveals how curcumin impairs cancer, https://www.sciencedaily.com/releases/2018/07/180709161543.htm
Gupta, S.C., Patchva, S. and Aggarwal, B.B. (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J, 15, 195-218.
Jäger, R., Lowery, R.P., Calvanese, A.V., Joy, J.M., Purpura, M. and Wilson, J.M. (2014) Comparative absorption of curcumin formulations. Nutrition Journal, 13, 1-8.
Lao, C.D., Ruffin, M.T., Normolle, D., Heath, D.D., Murray, S.I., Bailey, J.M., Boggs, M.E., Crowell, J., Rock, C.L. and Brenner, D.E. (2006) Dose escalation of a curcuminoid formulation. BMC Complement Altern Med, 6.